Guideline Creation for Cultivated Food | How Japan’s Subcommittee Is Building the Path Toward Safe Implementation
Megumi Avigail Yoshitomi
Please note that the content of this article does not represent the opinions of any individual member company of the Japan Association for Cellular Agriculture (JACA), nor does it reflect their collective consensus. It is solely the personal opinion of the author. There may be errors of interpretation in the course of organizing the information; therefore, if you have any comments, questions, or observations, please feel free to contact us (see the inquiry page here). For authoritative and up-to-date information, please consult official documents and guidance issued by the relevant government authorities.

Currently, discussions on the sales procedures of cultivated foods in Japan, as well as on safe and reliable production management, are being conducted by the Consumer Affairs Agency’s “Food Sanitation Standards Council, Subcommittee on Newly Developed Foods” (hereinafter referred to as the Subcommittee).
Around this summer, there is a possibility that an interim draft of the guidelines on cultivated foods will be released, so I would like to summarize the content of the discussions in the Subcommittee held so far before then.
Table of Contents
- How far have the discussions progressed?
- What happened in the past discussions?
- What are the key points of the upcoming discussions?
- Conclusion
How far have the discussions progressed?
As I understand it, the Subcommittee has been engaging in discussions along the following lines.
1. Discussion procedures and overview status
- The draft guidelines are being examined mainly by a working group centered on the National Institute of Health Sciences (NIHS). My understanding is that the working group first prepares a draft, which is then reviewed by the Subcommittee.
- It has been suggested that an “interim draft” may be presented to the Subcommittee by the summer of this year.
- As of July 25, 2025, the current progress is that hazards have been identified and organized (although risks have not yet been systematically assessed). Going forward, checkpoints for ensuring safety with respect to each hazard are expected to be established, after which a consolidated document will be prepared.
- It remains unclear whether the “interim draft by summer” refers to the organization of hazards mentioned above.
- It is also not clear when a preliminary version of the consolidated document will be ready.
- At the same time, discussions are also taking place on how to design the regulatory framework (i.e., the rules).

https://www.caa.go.jp/policies/council/fssc/meeting_materials/assets/fscc_cms105_250221_01.pdf
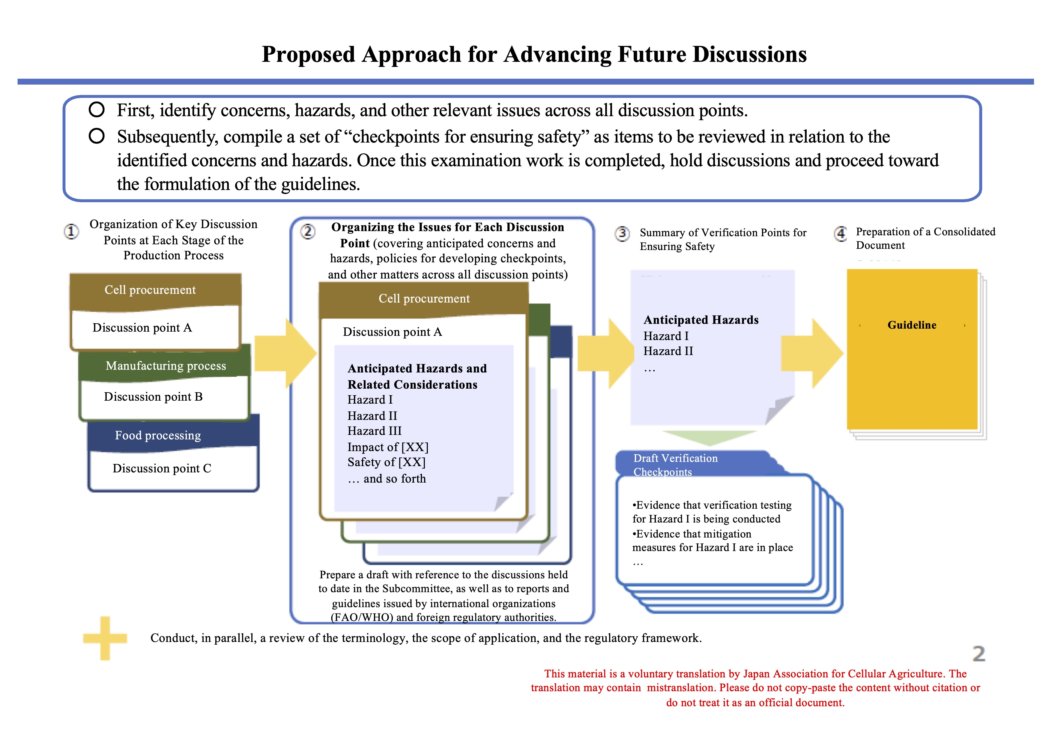
https://www.caa.go.jp/policies/council/fssc/meeting_materials/assets/fscc_cms105_250725_3.pdf
(As the links are as of July 29, 2025, there may have been updates since then.)
2. Discussion of the safety aspects
Here is a translated slide summarizing the government’s perspective on the safety of cultivated food.
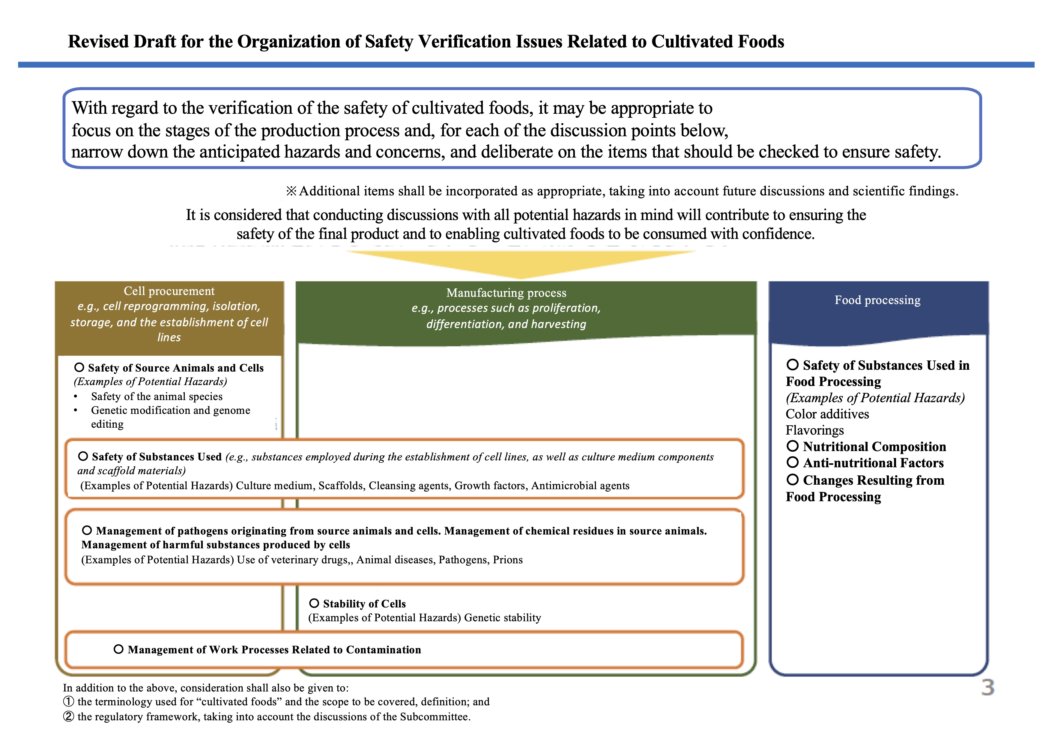
https://www.caa.go.jp/policies/council/fssc/meeting_materials/assets/fscc_cms105_250725_3.pdf
(As the links are as of July 29, there may have been updates since then. )
Here is the list of hazards and the management policy draft that was proposed during the Subcommittee on 25th July, 2025.
This material is a voluntary translation by JACA. The content here has been translated using AI, and it may contain mistranslations. It is provided for reference purposes only.
What happened in the past discussions?
Although somewhat dated, here is a compiled set of links containing minutes and summaries from past discussions held by the relevant government agencies.
https://jaca.jp/2025/07/7937/ (Please refer to the table in the linked article)
For those who wish to carefully verify the original primary sources, I encourage you to make use of it.
The discussions appear to have the following characteristics:
- First, on April 1, 2024, the administrative responsibility for food sanitation standards was transferred from the Ministry of Health, Labour and Welfare (MHLW) to the Consumer Affairs Agency. As a result, the body responsible for operating the Subcommittee (and the primary point of contact) changed.
- The treatment of this category within the Subcommittee meeting agendas has also evolved over time. Initially, as I recall, it was listed under “Other” among many topics, and only a limited amount of time was allocated for its discussion.
- In addition, around the time of the transfer of administrative responsibility for food sanitation standards, the “red yeast rice issue” arose. Consequently, it seems natural that during the first half of FY2024 (ending March 2025), cultivated foods did not appear on the Subcommittee’s agenda. However, starting with the meeting on November 18, 2024, cultivated foods began to be treated as the first discussion topic, and, as I recall, a significant amount of time started to be allocated to it as a top priority item.
- Although there have only been four Subcommittee meetings since FY2024, making direct comparison versus meetings until the end of FY2023 somewhat difficult, the frequency of participation from other government ministries and agencies has become high after FY2024. From the perspective of the Consumer Affairs Agency, this suggests an increased commitment to cross-ministerial discussions.
In addition, the Subcommittee admin office’s basic approach for making safety guidelines was presented during their meeting in February 2025. You can find the meeting materials used at that committee from the link below.
What are the key points of the upcoming discussions?
Below, I have summarized my personal views on which aspects to pay attention to when following the government’s future discussions. The items listed below focus primarily on those that are likely to influence when the first domestic case of sales will occur.
1. Granularity of the Guidelines
- What level of detail will be required from developers when compiling safety-related information?
- Will there be detailed instructions on the methods for obtaining each piece of safety information?
2. Scope of the Guidelines
- What balance will be struck between the responsibilities of companies and the areas in which the government verifies and collects information?
- Will the guidelines be updated frequently, and if so, what kind of procedures will be in place for such updates?
3. Structure and Other Considerations
- If areas for government verification and information collection are established, what will the structure and level of approval look like?
- Which ministry or agency will be responsible?
- How many personnel will be involved?
- Will it be a notification system, an approval system, compliance with guidelines, or something else?
- How will consistency with existing laws be ensured?
Will the cell-culture process be considered as the production of a food ingredient, or be regarded as food manufacturing? The ministry responsible may differ depending on this interpretation.
- After the Subcommittee formulates the guidelines, how much adjustment and implementation time will be needed before companies move forward with sales?
- What measures will be taken for risk communication with consumers?
Conclusion
At JACA, we are working to create a framework that enables cellular agriculture technologies to be safely and confidently integrated into Japanese society. To this end, we actively engage in discussions with government bodies, dialogue with relevant ministries, and exchanges of views with a wide range of stakeholders, including academia and consumers.
For our member companies, we provide activity reports that are even more detailed and substantive than what is shared in this article. We especially welcome companies seeking collaboration with Japanese partners to join us and work together in shaping the future of food.


